Treating traumatic joint injuries now to prevent osteoarthritis later
Post-traumatic osteoarthritis (PTOA) is a life-altering outcome for many patients after serious ankle fractures. CG-001 is being developed to address the biological triggers of this disease at the time of surgical repair—offering the potential to protect cartilage and preserve mobility before the damage is irreversible.

Disability and Quality of Life measurements following ankle fracture have been shown to be equivalent to end-stage renal disease and congestive heart failure
ankle fractures each year
Americans affected by PTOA
in annual healthcare costs
The current standard of care isn't enough
Each year, an estimated 99,000 people in the U.S. sustain ankle fractures likely to lead to PTOA within two years. These patients face pain, disability, and quality-of-life impact equivalent to congestive heart failure (CHF) or end-stage renal disease (ESRD).
Despite advances in surgical techniques, the risk has remained unchanged for decades because surgery repairs the bone but not the oxidative stress cascade that destroys cartilage.
of all surgically repaired ankle fractures manifest as PTOA, at an average of two years post-trauma

A novel, first-in-category approach targeting a primary cause of PTOA
CG-001 is an intra-articular injection currently being evaluated in clinical testing for administration during standard ORIF surgery (Open Reduction and Internal Fixation), which is the current standard of care for traumatic ankle fracture injuries.
It is designed to block the surge of excess reactive oxidant species (ROS) caused by injury—an upstream driver of cartilage degeneration—validated in translational large-animal models with sustained protective effects. The treatment has been developed to be simple to administer, integrates seamlessly into surgical workflows, and focuses on modifying the disease process, not just managing symptoms.
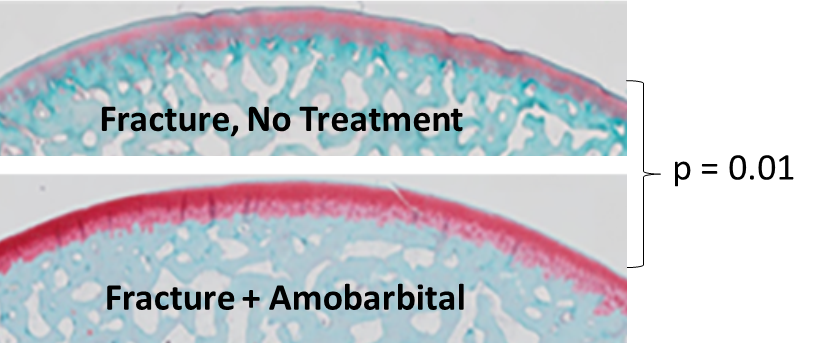
Single amobarbital dose administered by intra-articular injection acutely after fracture dramatically slowed cartilage degeneration, a hallmark of PTOA progression.

CartilaGen is building a solution for those who treat—and those who invest in—better outcomes
For orthopedic surgeons and trauma specialists, CG-001 will present a novel therapeutic option with the potential to reduce long-term joint degeneration with no added complexity in the operating room.
For investors and partners, CG-001 represents an exciting, first-in-category therapeutic with strong IP protection, clear unmet need, and significant market potential across civilian, sports, and military injury settings.
CartilaGen is in a position to revolutionize the standard of care in this field
- CG-001 is the only therapy in clinical development specifically for PTOA prevention after ankle fractures
- Phase 1 trial completed with no adverse safety findings
- Department of Defense funding secured to support planning Phase 2 efficacy trial
- IP protection through 2044 and beyond
- Clinical development led by experienced team of drug developers and a world-class scientific advisory board
Our planned trial sites have been selected to include recognized leaders in orthopedic care and research






Take the next step toward better outcomes
Whether you’re a surgeon looking for more information on how to help support the future clinical development of CG-001, or an investor ready to support a first-of-its-kind therapy, we would like to meet you.
